Subangstrom resolution X-ray structure details aquaporin-water interactions (#31)
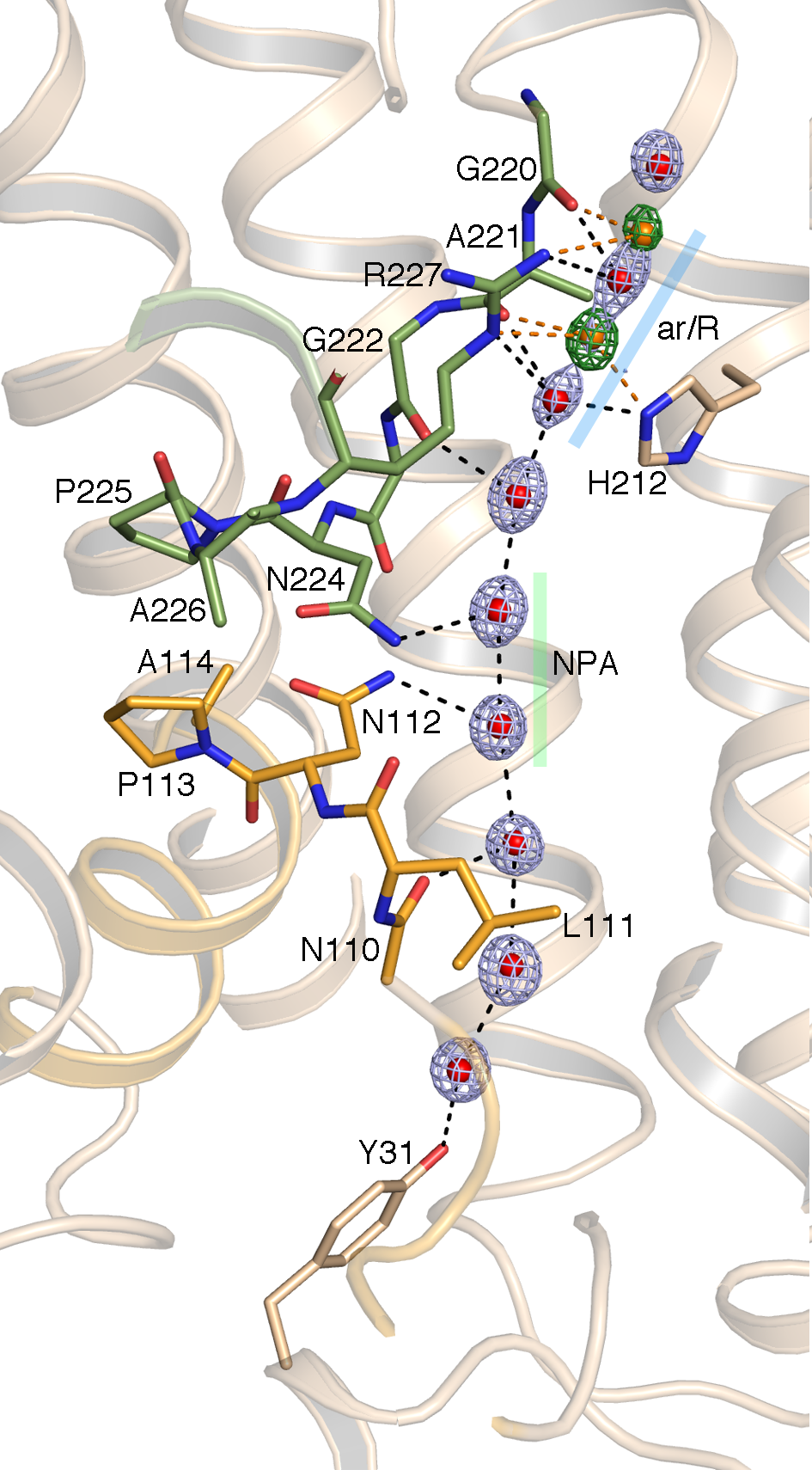
Aquaporins are proteins that facilitate transport of water across biological membranes. They must be selective for water, without binding it so tightly as to impede transport, and they must prevent proton transfer by rapid exchange between hydrogen-bonded water molecules. Crystal structures have been determined for many aquaporins from a wide range of organisms. All structures show a conserved fold composed of six transmembrane α helices arranged in a homotetramer, with each monomer acting as an independent water channel. Aquaporins have an inverted repeat structure, where the amino- and carboxyl-terminal halves of the protein share a similar structural fold and are related by a two-fold symmetry.
We have resolved the structure of yeast aquaporin 1 at ultrahigh resolution (0.88 Å, a record for membrane proteins) and in doing so we could experimentally observe previously unresolved aspects of water transport. Many aquaporin structures have been solved at high resolution (<2 Å), but the precise hydrogen bonding between key amino acids and the water molecules emerges only at subangstrom resolution. This unprecedented level of detail for a membrane protein provides a foundation for future studies to investigate the interplay between protein structure and water flux.
- Subangstrom resolution x-ray structure details aquaporin-water interactions. Kosinska Eriksson U, Fischer G, Friemann R, Enkavi G, Tajkhorshid E, Neutze R, Science 340: 1346-9 (2013).
- Watch Water Flow. Abramson J, Vartanian AS, Science 240: 1294-95 (2013)
 PROTEINS 2015*
PROTEINS 2015*